Activating Cancer Drugs with the Flick of a Light Switch
What if you could attach a drug to a tiny particle that acted as a “vehicle” for the delivery of your drug to a target cell in the human body, and then, only after safe delivery, control release of the drug from its vehicle with the flick of a light switch? Remotely triggerable drug delivery systems have been a goal for many years in the biomedical and nanotechnology fields of research. The idea is that if drugs can be targeted and delivered only to the cells that need them, required doses of the drugs for successful therapy are reduced, as are the potential side effects of such drugs on healthy tissues and cells. Take chemotherapeutic drugs for instance – they are traditionally only roughly targeted to quickly dividing cells, meaning that any quickly dividing cells within the human body, hair follicle cells for example, are affected as well as cancer cells. More precise targeting of chemotherapeutics would decrease the toxicity of these drugs as well as increase their therapeutic efficiency within cancerous regions.
Some remotely triggerable drug delivery systems are based on light, others on ultrasound or magnetic fields, all of which can penetrate variable distances within the human body without the need for invasive procedures. The idea is to only release drugs inside of the cells that need them, the cells that are targeted by the drug delivery vehicle.
This was the premise of my research as master’s degree student in the Biological & Agricultural Engineering program at Louisiana State University. This was the premise, but the practice of this technology took place inside of a laboratory petri-dish with cultured human cervical cancer cells, and should be interpreted in light of this setting.
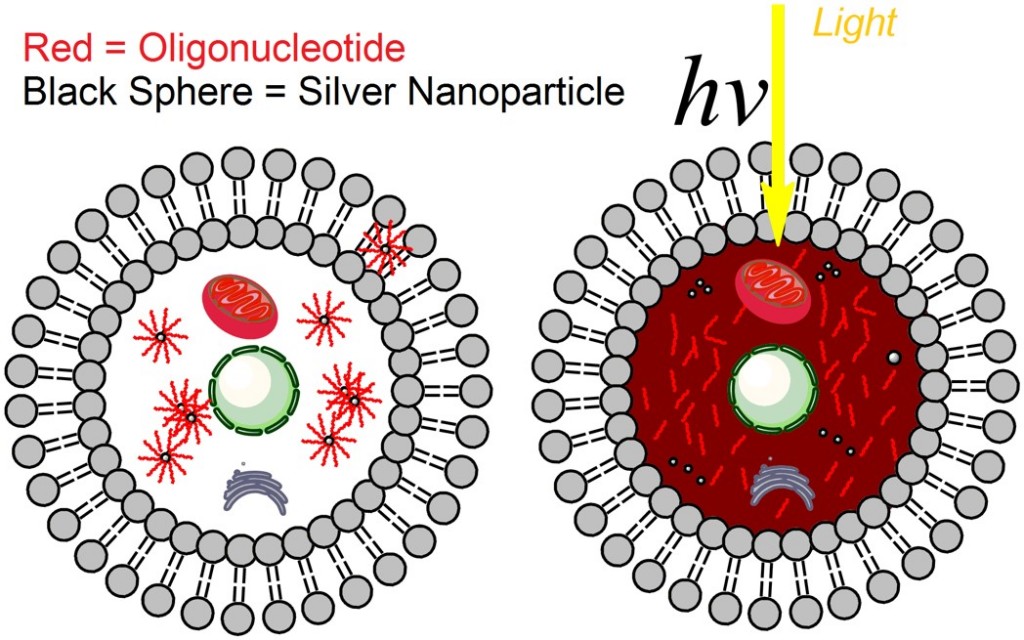
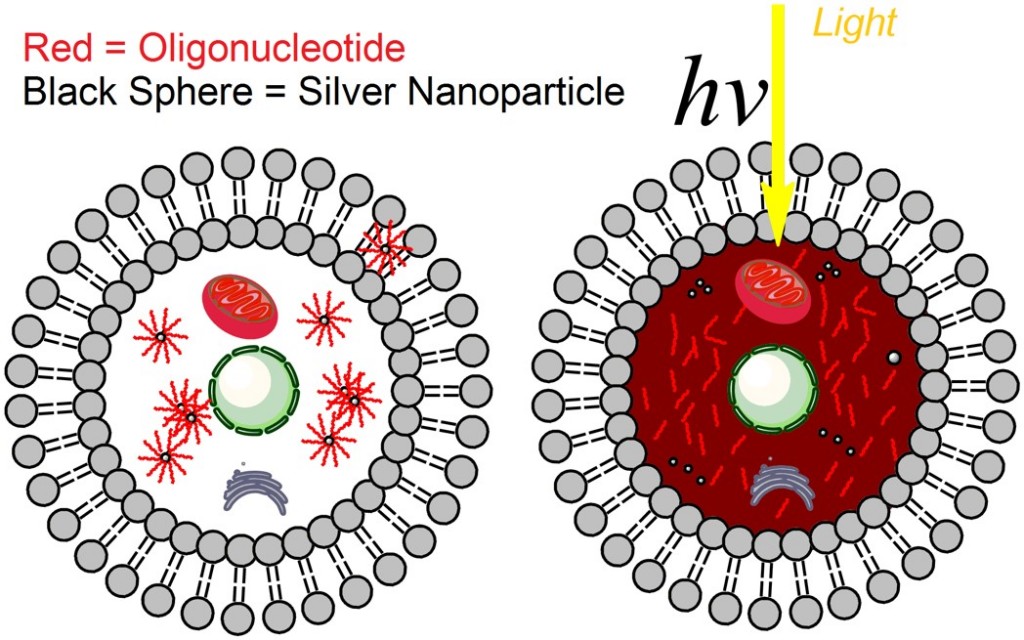
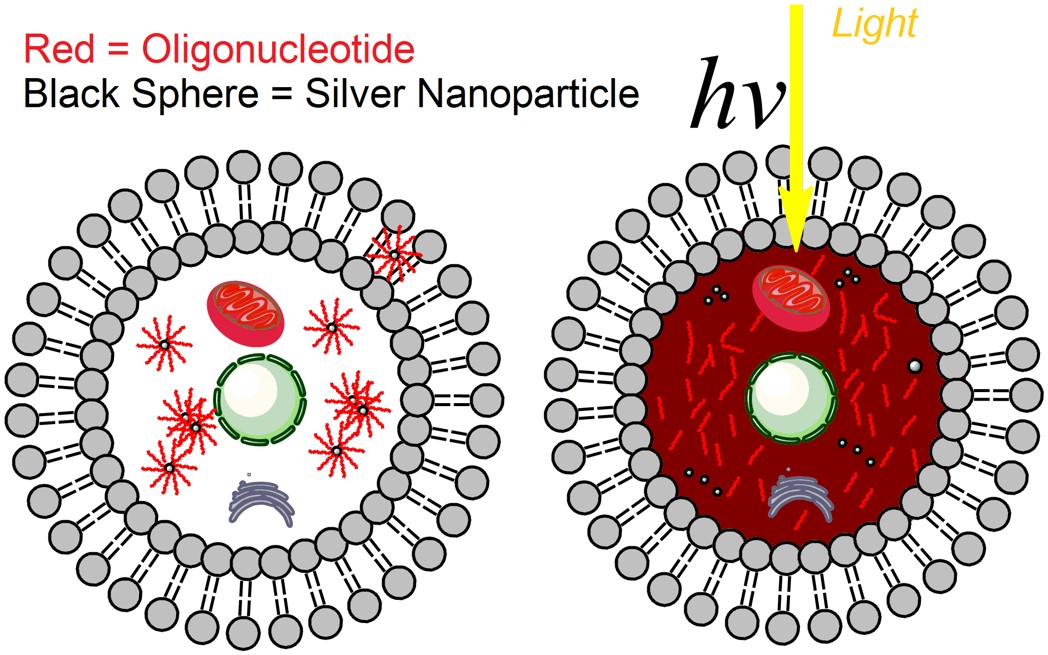
In research published this March in ACS Nano, myself and my research colleagues and professors at LSU demonstrated the ability of tiny silver particles, or silver nanoparticles, to safely delivery drug payloads in human cancer cells in a laboratory environment. Silver nanoparticles interact with light in special ways that other nanoscale materials don’t, amplifying light signals in particular regions, thus potentially providing unique opportunities to use light as an improved remote drug-release trigger. With the ‘light switch’ turned off, the drug remains on the nanoparticle surface. When the ‘light switch’ is turned on, and the cells containing the nanoparticles are exposed to ultraviolet light, the drug is released and becomes active within the target cells.
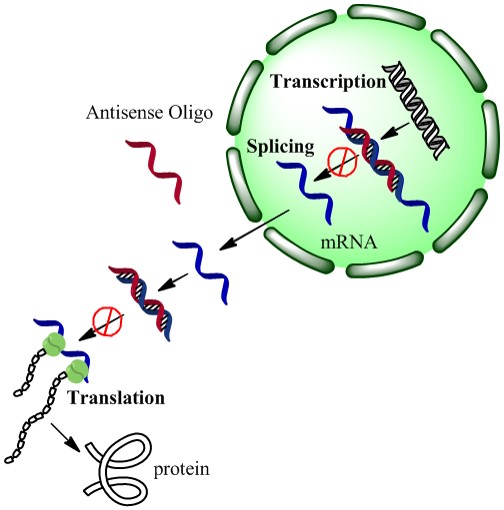
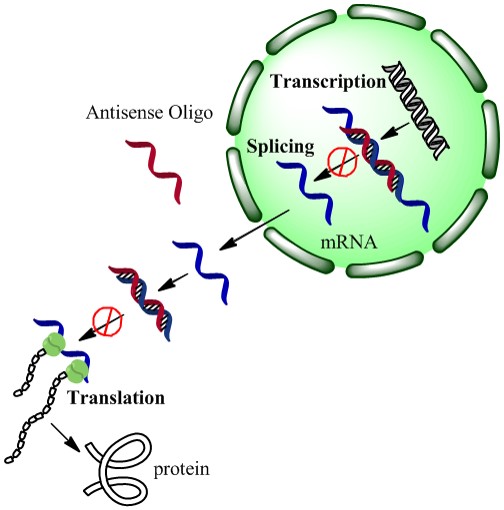
We also demonstrated that when our drugs were on the nanoparticle surface, not yet released by exposure to light, the drugs were more stable to enzymes in the cellular environment that can degrade the drug into useless fragments. In this case, our drugs were strands of DNA that can turn off, or at least turn down, the expression of particular genes in cancer cells. The drugs were also not active until they were released from the nanoparticle surface with a light trigger – we call this a “caged” effect, as if the drugs are in an inactive ‘cage’ until they are released from their nanoparticle vehicle.
A fancy way of explaining these results:
“A new platform for enhanced delivery of antisense therapeutics and photoactivated gene silencing with in situ detection of activation could augment gene expression analysis, clinical antisense therapies and the study of genetic diseases. Our designed Silver-nanoparticle-oligonucleotide conjugates display desirable properties as gene delivery agents: enhanced cellular uptake, increased oligonucleotide stability or therapeutic lifetime, and a steric caged effect of the nanocarrier with light-inducible activation.”
Of course, our study has important limitations. These include the fact that silver nanoparticles can have potentially adverse effects on human tissues if used in high amounts, although silver nanomaterials are currently being widely investigated and used as antibacterial agents and biomedical coatings. Also, the ultraviolet light trigger used in our study to release drugs from their nanoparticle vehicles can’t penetrate as deeply into the human body as visible and infrared light can. Actually, this is one reason for using silver nanoparticles in the first place, in the hopes that the special light properties of these materials can make drug release with deeper-penetrating light possible.
But nanoparticles are still a hot topic in drug delivery research – a quick Google Scholar search will reveal many recent research studies in this area. Viral nanoparticles for drug delivery are a particularly interesting case, using natural virus-derived materials as systems for improved drug delivery and cancer treatment.
Have any questions about nanotechnology in medicine? Ask away! This was my specialty before I became a science communication nerd.
Silver Nanoscale Antisense Drug Delivery System for Photoactivated Gene Silencing, Paige K. Brown, Ammar T. Qureshi, Alyson N. Moll, Daniel J. Hayes, and W. Todd Monroe, ACS Nano 2013 7 (4), 2948-2959.